Losing organs and tissues is a significant human health problem. Tissue engineering is well-known as a new approach for repair, replacement or regeneration of tissues and organs that are damaged through traumatic injuries, aging or illness.
In the last two decades, tissue engineering methods have had very successful outcomes in regenerating skin cartilage and, more specifically, bone. The number of bone fractures in the U. S. is more than 6.2 million. Unfortunately, the healing procedure of 10% of this number is not successful due to non-union or delayed union. Different methods have been utilized for this huge demand of bone healing such as autografts, bone allografts and metallic implants. But they have major disadvantages such as limited supply and donor site morbidity for the case of autografts, and cost issues and risk of disease transmission for the case bone allografts. Metallic implants may also become movable and loose over time. In addition, they are not self-repairing material and do not adjust with alterations in physiological conditions. The deficiencies of the above approaches have made bone tissue engineering very popular in the last decade.
Achievements
I am collaborating with the biomaterials group at HRC in this area. We recently formed a Nano-Bio consortium to connect the research in nano science and biotechnology. Usually the faculties who are in the area of nanotechnology are not aware of possibility of collaboration with faculties in the bio research and vice versa. Also, they are typically not familiar with the facilities and small equipment of each other laboratories that can be used for novel multidisciplinary research. This consortium helps to enhance faculties’ knowledge through networking so they can team up on grant proposals, collaborating in research projects, and publishing common papers. Many scientists from different universities joined the consortium.
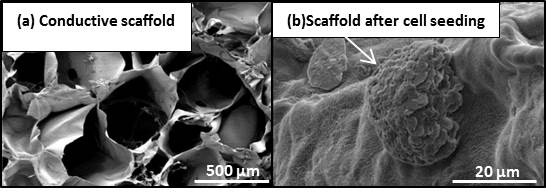
Figure 1: a) An example of a conductive scaffold produced in our laboratory. The SEM image shows its porosity and microstructure.
This scaffold is a composite of PEDOT conductive Polymer (Gelatin, Chitosan, Hialuronic Acid and PEDOT). b) The scaffold loaded with
Hepatocyte cell-line GS-5.
Representative Publications
- Surface modification of stainless steel orthopedic implants by sol–gel derived ZrTiO4 and ZrTiO4–PMMA coatings, E. Salahinejad, M.J. Hadianfard, D.D. Macdonald, S. Sharifiasl, M. Mozafari, K.J. Walker, A. Tahmasbi Rad, S.V. Madihally, D.Vashaee, L. Tayebi, Journal of Biomedical Nanotechnology, Vol. 9, 1–9, 2013, 1550-7033/2013/9/001/009, DOI:10.1166/jbn.2013.1619 (2013)
- Surface microstructure and in vitro analysis of nanostructured akermanite (Ca2MgSi2O7) Coating on biodegradable magnesium alloy for biomedical applications, Mehdi Razavi, Mohammadhossein Fathi, Omid Savabi, Batoul Hashemi Beni, Daryoosh Vashaee, Lobat Tayebi, Colloids and Surfaces B: Biointerfaces , DOI: 10.1016/j.colsurfb.2013.12.011
- Influence of annealing temperature on the structural and anti-corrosion characteristics of sol–gel derived, spin-coated thin films, E. Salahinejad, M.J. Hadianfard, D. Vashaee, L. Tayebi, Ceramics International, Volume 40, Issue 2, March 2014, Pages 2885–2890
- Effect of precursor solution pH on the structural and crystallization characteristics of sol–gel derived nanoparticles, E. Salahinejad, M.J. Hadianfard, D. Vashaee, L. Tayebi, Journal of Alloys and Compounds, (In Press, Accepted- 2013)
- A new double-layer sol–gel coating to improve the corrosion resistance of medical-grade stainless steel in a simulated body fluid, E. Salahinejad , M.J. Hadianfard , D.D. Macdonald , S. Sharifiasl , M. Mozafari , D. Vashaee , L. Tayebi, Materials Letters, DOI: 10.1016/j.matlet.2013.01.111, Volume 97, Pages 162–165 (2013)
- Nanostructured zirconium titanate fibers prepared by particulate sol-gel and cellulose templating techniques, P. Rouhani, E. Salahinejad, R. Kaul, D. Vashaee , L. Tayebi, Journal of Alloys and Compounds, Volume 568, 15 August 2013, Pages 102–105, DOI: 10.1016/j.jallcom.2013.03.142 (2013)
- Multilayer bioactive glass-zirconium titanate thin films for bone, Masoud Mozafari, Erfan Salahinejad, Vahid Shabafrooz, Mostafa Yazdi Mamaghani, Daryoosh Vashaee, Lobat Tayebi, International Journal of Nanomedicine, 2013:8 1665–1672, DOI : 10.2147/IJN.S42659, (2013)
- Synthesis and Characterization of Al-SiC-Al2O3 Metal Matrix Composite Powder, M. Karbasi, M. Razavi, M. Taheri, D. Vashaee , L. Tayebi, Micro and Nano Letters (Accepted, 2013)
- Hybrid macroporous gelatin/bioactive-glass/nanosilver scaffolds with controlled degradation behavior and antimicrobial activity for bone tissue engineering, M. Yazdimamaghani, D. Vashaee, S. Assefa, K. J. Walker, S. V. Madihally, G. A. Köhler, L. Tayebi, Journal of Biomedical Nanotechnology (In Press, 2013)
- Synthesis and Characterization of Encapsulated Nanosilica Particles with an Acrylic Copolymer by In Situ Emulsion Polymerization Using Thermoresponsive Nonionic Surfactant, M. Yazdimamaghani, T. Pourvala, E. Motamedi, B. Fathi, D.Vashaee, L. Tayebi, Materials (Accepted, 2013)
- Electrophoretic deposition of nanostructured akermanite coating on biodegradable magnesium implants: in vitro bio-corrosion and bioactivity behavior, Ceramics International M. Razavi, M. Fathi, O. Savabi, S. M. Razavid, B.H. Beni e, D. Vashaee, L.Tayebi, Ceramics International (Accepted, 2013)
- Electroconductive nanocomposite scaffolds: a new strategy into tissue engineering and regenerative medicine,M.Mozafari, M. Mehrayin, D. Vashaee, L. Tayebi Book Chapter in “Nanocomposites – New Trends and Developments”, ISBN 978-953-51-0762-0 (2012)
- Nanofibrous scaffolds for tissue engineering applications: state-of-the-art and future trends, L. Tayebi, M. Mozafari, V. Shabafrooz, M. Yazdimamaghani, D. Vashaee, Editor: Qing Liu, Book Chapter in: Tissue Regeneration: Where Biology Meets Nano Structures. 3D Biotek, LLC, North Brunswick, New York, USA (In press, 2012)
- Zirconium titanate thin film prepared by an aqueous particulate sol-gel spin coating process using carboxymethyl cellulose as dispersant, E. Salahinejad; M. J. Hadianfard; D. Macdonald; M. Mozafari; D. Vashaee; L. Tayebi, Materials Letters, DOI: 10.1016/j.matlet.2012.08.013 (2012)
- Aqueous sol-gel synthesis of zirconium titanate (ZrTiO4) nanoparticles using chloride precursors, E. Salahinejad, M.J. Hadianfard, D. Vashaee, D. Macdonald, I. Karimi, L.Tayebi, Ceramics International, DOI: 10.1016/j.ceramint.2012.04.064 (2012)
- Multilayer zirconium titanate thin films prepared by a sol-gel deposition method, E. Salahinejad; M. J. Hadianfard; D. Macdonald; M. Mozafari; D. Vashaee; L. Tayebi, Ceramics International,10.1016/j.ceramint.2012.07.058 (2012)
- Current opinion in tissue engineering microscopy techniques, L. Tayebi, A. Nozari1, D. Vashaee, M. Mozafari, Book Chapter in “Current microscopy contributions to advances in science and technology” Microscopy Book Series Volume # 5, Formatex 2012, ED.: A. Méndez-Vilas, PP (591-601)
- Self-standing nanostructured zirconium titanate fibers prepared by particulate sol-gel and cellulose templating technique, P. Rouhani, E. Salahinejad, R. Kaul, D. Vashee, L. Tayebi, Journal of Alloys and Compounds, (In press, 2013)
- Surface modification of stainless steel orthopedic implants by sol–gel derived ZrTiO4 and ZrTiO4–PMMA coatings, E. Salahinejad , M.J. Hadianfard , D.D. Macdonald , S. Sharifiasl , M. Mozafari , K.J. Walker, A. Tahmasbi Rad , S.V. Madihally ,D. Vashaee, L. Tayebi, Materials Today Virtual Conference: Nanotechnology, 2012, December 11th – 13th, Web-based Conference Sponsored by Elsevier Ltd.
- Performance enhancement of electrospun carbon fibrous nanostructures, Vahid Shabafrooz , Masoud Mozafari, Daryoosh Vashaee, Lobat Tayebi, Materials Today Virtual Conference: Nanotechnology, 2012, December 11th – 13th, Web-based Conference Sponsored by Elsevier Ltd.
- Antibacterial activity and stability of silver nanoparticles in gelatin matrix papered through a rapid room temperature chemical route, Mostafa Yazdi Mamaghani, Gerwald A. Köhler, Masoud Mozafari, Senait Assefa, Vahid Shabafrooz, Daryoosh Vashaee, Lobat Tayebi, Materials Today Virtual Conference: Nanotechnology, 2012, December 11th – 13th, Web-based Conference Sponsored by Elsevier Ltd.
- Robust carbon nanofibrous mats from polyacrylonitrile conductive polymer, V. Shabafrooz, M. Mozafari, M. YazdiMamaghani, D. Vashaee, L. Tayebi, Materials Today Conference: Nanotechnology, 2012, December 11th – 13th, Web-based Conference Sponsored by Elsevier Ltd.
- Synthesis and antibacterial activity of gelatin\nanosilver scaffolds intended for soft tissue engineering applications, Mostafa Yazdi Mamaghani, Gerwald A. Köhler, Masoud Mozafari, Senait Assefa, Vahid Shabafrooz, Daryoosh Vashaee, Lobat Tayebi, Materials Today Virtual Conference: Nanotechnology, 2012, December 11th – 13th, Web-based Conference Sponsored by Elsevier Ltd.
- Degradation behavior of hyaluronic acid-gelatin scaffolds intended for intestine tissue engineering,V. Shabafrooz, M. Mozafari, M. YazdiMamaghani, D. Vashaee, L. Tayebi, Materials Today Conference: Nanotechnology, 2012, December 11th – 13th, Web-based Conference Sponsored by Elsevier Ltd.
- Thermal Stability of Lead Sulfide Nanocrystals Synthesized Through Green Chemical Route, Masoud Mozafari, Fathollah Moztarzadeh, Daryoosh Vashaee, Lobat Tayebi, IEEE Green Technologies Conference 2012 – Energy Generation & Storage Technologies, Tulsa, Oklahoma, April 19-20, 2012
- Effects of heat treatment on physical, microstructural and optical characteristics of PbS luminescent nanocrystals, Mozafari, F. Moztarzadeh, D. Vashaee, L. Tayebi, Physica E Low-dimensional Systems and Nanostructures, DOI: 10.1016/j.bbr.2011.03.031. (2012)
We have developed quite many different types of materials for bio medical application such as new ZrTiO4based thin films used in implants, nanocomposite scaffolds for bone tissue engineering, carbon nanofibrous mats from polyacrylonitrile conductive polymer, etc.
Our ongoing studies in this field are mostly focused on improving the physical characteristics of the scaffolds with respect to their biocompatibility, bioactivity, and biodegradability. It is known that electric stimulation has progressive influence in healing treatment. We are developing scaffolds with improved electrical conductivity that act as a novel tissue engineering substitute and as a new generation of scaffolds to repair critical defects (Figure 1). This class of scaffolds may have significant effects in the future of regenerative medicine.