Nanocomposites comprise of nanocrystals of different phases or composition, where crystallites and interfacial nanostructures form a disordered morphology. An interesting feature of these materials is that the energy disruption for both electrons and phonons can happen in a time scale that is smaller or comparable to the energy relaxation time. Therefore, the charge and energy carriers can maintain an energetically non-equilibrium distribution that can result in fundamentally different electrical and thermal properties than in the crystalline materials.
The change in energy spectra of electrons can significantly affect the transport parameters as the electrons are now placed at different positions in the energy band. Characteristics, such as the density of states, conductivity effective masses, and the degeneracy of the band, may all change significantly resulting in a material that would possess quite different electrical properties than its homogenous bulk counterpart.
Apart from energy band discontinuity of two materials and the space charge interface potential barriers, electrons may experience a potential barrier (or well) due to the structural rotation of the adjacent grains. For example, due to the crystallite rotation, an electron that is in the X valley of a crystallite may have a wave-vector (k) that is in direction of the L-point electron wave-vectors of the adjacent grain. However, if the L-point is at higher energy, say for a hypothetical material, and if the direction of the k-vector is not changed in transport, only the high energy electrons in the X valley may enter the L valley of the adjacent crystallite (Figure 1, X-L black line). Electrons that have entered the L valley may move across the crystallite and enter X valley in an adjacent crystallite (Figure 1, L-X red line). It would take a finite time for these electrons before they relax to the band edge at the X point, during which time, these electrons are at a significantly higher energy than their equilibrium state in a similar bulk material. If the crystallite is small enough, these electrons may enter the X valley of another crystallite before they are relaxed. Such cases affect the transport parameters, such as the Seebeck coefficient and the energy dependent scattering rates, from their equilibrium values.
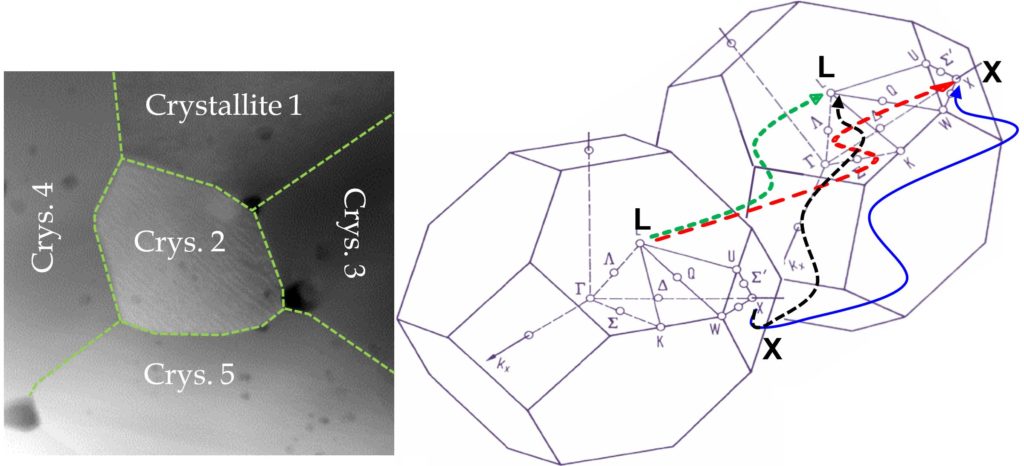
Figure 1: L-L and X-X lines demonstrate the equivalent intervalley scattering between adjacent grains. L-X line shows transition to a different valley that would disturb the energy spectra of the charge carrier.
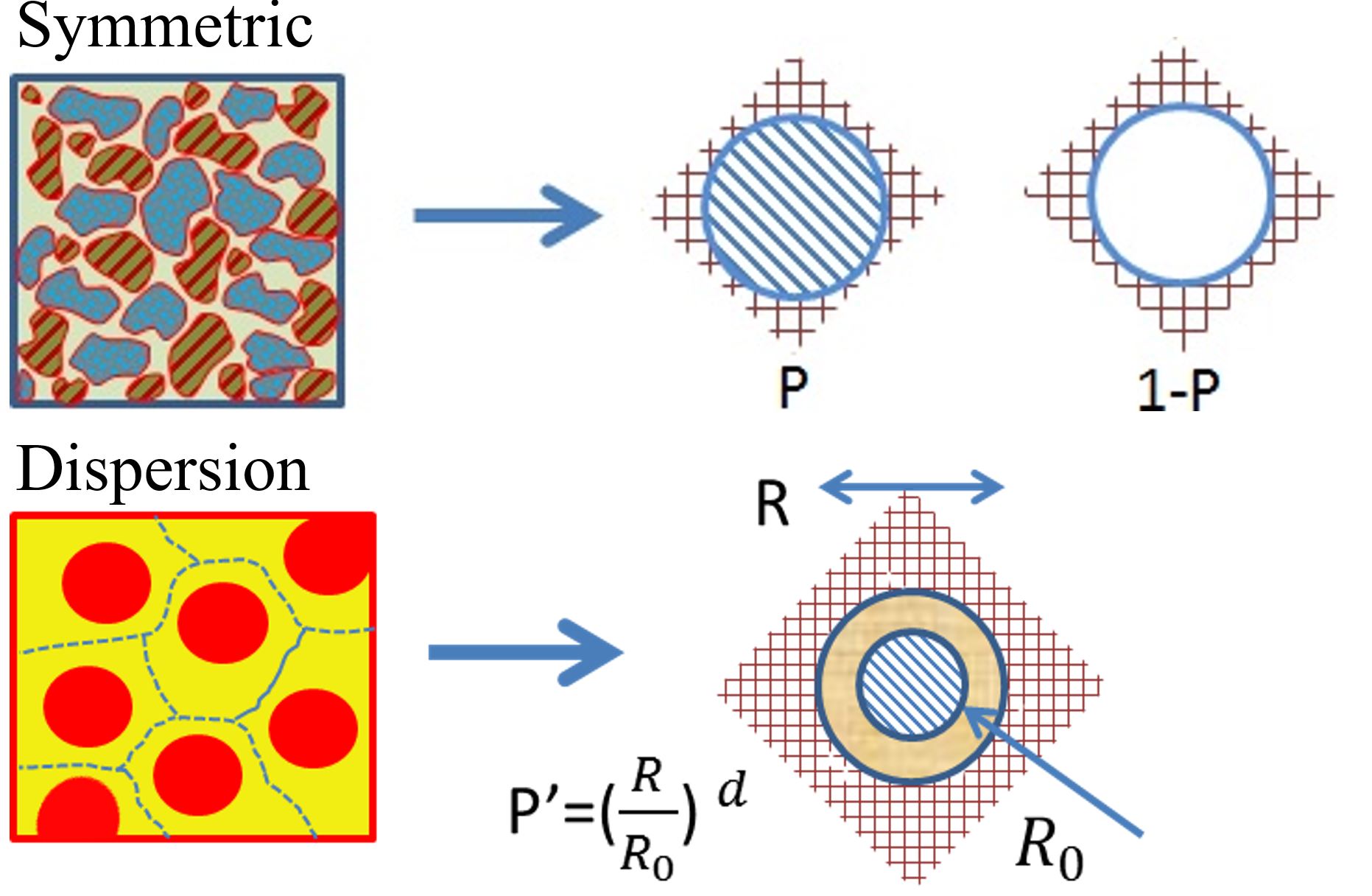
The most known structural units for disordered systems are: (1) symmetric and (2) dispersion structures, as depicted in Figure 2.
Symmetric microstructure: This nanostructure is symmetric in the statistical sense; i.e., an interchange of the components results in the same type of medium with interchanged volume fraction. In this case, there are two components and two structural units, each approximated by a sphere of one material component. Therefore, the volume fractions are x and 1–x. P and 1-P in Figure 1-top refer to the probability of the existence of each component, respectively.
Dispersion microstructure: For dispersion nanostructure, one component is the dispersed phase in the host or matrix component. In the dispersion nanostructure, as can be seen in the Figure 1-bottom, the medium includes similar structural units, each consisting of a dispersed component particle surrounded by a layer of the host component. These similar structural units can be approximated by a coated sphere. R is the radius for the whole unit, R0 is the radius for the dispersed particle, and P’ is its local volume fraction which is varying from one location to the next.
Different material processing steps are often taken to develop such materials such as induction melting, quenching, ball milling, plasma pressure compaction, filed induced de-crystallization, doping, alloying, etc.
Material Design and Discovery
Such new characteristics offer a landscape for discovering new material systems for engineering applications. For instance, maintaining the energy of hot electrons has been a long standing challenge in narrow band-gap photovoltaics (PV). A narrow band-gap PV material can harvest a larger part of the solar spectra; however, the electrons excited by high energy photons relax to the band edge giving up their extra energy to phonons. Therefore, the extra energy is wasted as heat.
A similar motivation exists for enhancing the power factor in thermoelectric materials. Instead of focusing on semiconducting materials with a highly asymmetric density of states close to the Fermi level to increase the average energy of charge carriers (hence the Seebeck coefficient), the multi-phase nanocrystalline approach offers a new way via the non-equilibrium transport of charge carriers. This method alleviates the fundamental trade-off between the electrical conductivity and the Seebeck coefficient. The high electron density compensates for the negligible contribution to the conductivity from the majority of the electrons that have energies below the top of the interface potential barrier.
This research should lead to the discovery of new three-dimensional (3D) material systems with fundamentally novel electrical and thermal transport properties compared with conventional bulk electronic materials. Although the influence of size quantization of the energy spectra in one- and two- dimensional structures has been investigated to some extent, understanding of charge and thermal transport properties in 3D nanocrystalline materials is still limited when the crystallite sizes shrink to lower than the carrier relaxation lengths. The results obtained are of interest for understanding a wide range of effects where electron and phonon transfer phenomena play a major role.
Technological Applications
Historically Radioisotope Thermoelectric Generators (RTG) have been used to power NASA spacecrafts. Solar energy is not available deep in the space. Mechanical engines are not reliable for long time missions either as they need maintenance. Thermoelectric generators are solid state devices with no moving part and can reliably work for decades. Voyager I was sent to space in 1977 and is now travelling in “Interstellar space”. The RTG that has powered Voyager I has been constantly working since then without single fault. There has no mechanical engine with such a high reliability. RTGs have also been used to power the Curiosity rover on Mars.
Today nearly 60% of the useful energy is wasted as heat. In the U.S. alone, fuel for vehicles costs billions of dollars a year, with most of the power wasted as heat due to low efficiency of combustion (Figure 5). Light-duty passenger, vans, SUVs waste nearly 80 billion gallons of gasoline in the exhaust and radiator. Heavy-duty vehicles waste nearly 19 billion gallons of diesel. Similar energy is wasted at various industries equivalent to nearly 80 billion gallons of gasoline. Thermoelectric generators can be used convert the low grade waste heat directly into electricity. It is expected that thermoelectric generators should play an important role is recovering significant amount of wasted heat helping to reduce consumption of fossil fuels and to reduce the greenhouse gas emissions.

Figure 5: Nearly 60% of the world’s useful energy is wasted as heat. Thermoelectric generators can recover some of the wasted fuel energy and convert it from heat into useful electric power.